Like On-the-Skin Labs, Sweat Sensors Assess Biomarkers
This is the first in a series of in-depth articles about research aimed at Improving Access to Cancer Care for Personalized Medicine led by American Cancer Society (ACS) staff or funded scientists.
Part I: Sweat Sensors
ACS grantee uses the Internet of Everything to design compact, all-in sweat sensors that collect live data continuously, painlessly—and eventually, at home.

This example of a sweat sensor designed in the Gao Lab demonstrates how unobtrusive the device is due its flexibility, low profile, and compact technology. FPCB means flexible printed circuit board. Photo Credit: Wei Gao
Biomedical engineer Wei Gao, PhD, has revolutionized the concept of a sweatshop. In his modern world sweatshop—the Gao lab at California Institute of Technology (Cal Tech)—scientists build small, wearable devices that analyze sweat. It may sound messy or a bit gross, but the biosensors they develop collect valuable chemical information from teeny bits of sweat. The reason is a noble one. His complex and reusable sensors have the promise of helping a lot of people. Gao’s lab team has already published studies about sensor prototypes that might change the lives of people with diabetes, gout, obesity, stress, and more. Now, he’s supported by an American Cancer Society (ACS) grant to study sweat sensors for people with certain types of leukemia. HIs lab's technological platform is a valuable tool to help realize a future where personalized and precision medicine is the standard of care. Not surprisingly, the sweat biosensors and Gao’s lab team are getting a lot of attention from researchers and clinicians.
Researchers and clinicians anticipate that precision medicine will eventually allow them to tailor prevention and treatment strategies for each person based on his or her unique physiological and behavioral characteristics. For instance, each strategy could be adapted for a person’s genome sequence, microbiome composition, health history, lifestyle, and diet.
In Plain Words
Want to better understand precision medicine, biomarkers, or the Internet of Everything? See Understanding Cancer Research Terms.
That advancement in precision medicine relies directly on biomarkers, the biological indicators of disease. Scientists and doctors already find and measure many biomarkers—glucose, nutrients, genes, proteins, and hormones to name just a few. Several molecular biomarkers are already used to help cancer patients, like the protein HER2 and the hormone ER. Still, there are no known biomarkers for numerous health conditions including Alzheimer’s disease, epilepsy, and long COVID-19.
“I have an Apple Watch that I often wear during exercise. But all the commercially available varieties of wearable health devices are not sufficient. We want more. We want more data, a different type of data—not only my heart rate or number of steps. Wanting that is my motivation to bridge the gap, to develop a device with variables that can not only give us physical information but also give us information at a molecular level—at the biomarker level. That vision is what inspired me to become a biomedical engineer.”
–Wei Gao, PhD, ACS grantee from Cal Tech in Pasadena

Sweat informs scientists about the small stuff, and sensors allow for the big data that can be shared over the internet via a networked connection of objects known as the Internet of Things.
In a recently published study supported by the American Cancer Society, Gao explains how discovering new biomarkers and validating them can be an expensive, time-consuming process of trial and error. That’s especially true when investigators look for biomarkers by using the traditional process: collecting and analyzing blood samples. Wearable biochemical sensors are an emerging technology that offer a less invasive, more convenient, and less expensive method to screen for potential biomarkers. But so far, wearables mostly monitor physical markers, like heart rate or oxygen saturation.
Gao’s lab doesn’t collect blood to measure biomarkers. They use “alternative body fluids” that are less invasive to collect from people—sweat, saliva, and tears. “Sweat has hundreds of chemical compounds, and it changes depending on a person’s health,” he explains. Many biomarkers in sweat have a good correlation with their concentration in blood. These biomarkers can be used to track a person’s metabolism, stress, and to help understand what is happening during certain sports and with certain health conditions.
Other scientists have made sweat sensors, but Gao’s lab has taken them to a much higher level. The design has involved the collective expertise of a highly interdisciplinary team of over 30 people for about 4 years. The team includes materials scientists, chemists, and mechanical, electrical, chemical, and biomedical engineers.
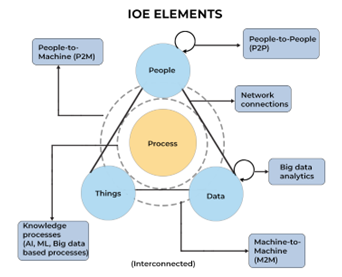
People are one element of the Internet of Everything (IoE). This 21st century digital system is an intelligent network of people, process, data, and things (objects, devices, appliances, and so forth that use implanted sensors to communicate across the network). This interconnectedness allows data from all devices to be analyzed and processed together.
IoE works in sync with technologies such as artificial intelligence, machine learning, and the cloud. These processes ensure the correct information is relayed to the right destination across the network and to leverage data faster.
A subset of the IoE is “things,” or the Internet of Things, which includes those devices with sensors or other technology that are connected by a network and that can exchange data over the internet without human intervention. A pacemaker, for example, is an Internet of Things(IoT) device, as are smart thermostats, home security systems, and self-driving cars.
Crash Course on Molecular Biomarkers
According to the US Food and Drug Administration (FDA), there are 7 categories of biomarkers.
Susceptibility biomarkers signal the potential, or risk, a person has to develop a disease before they have symptoms. Low-density lipoprotein (LDL) cholesterol is a susceptibility biomarker for heart disease.
Diagnostic biomarkers help identify and diagnose conditions. High glucose levels are a diagnostic biomarker for diabetes.
Response biomarkers help confirm whether a prevention method or treatment worked. After vaccination, antibodies are the response biomarkers.
Safety biomarkers help identify possible toxic responses to treatment or harmful environmental exposures. Creatinine is a safety biomarker for drugs that can potentially affect the kidney.
Prognostic biomarkers indicate the chances of a disease progressing or recurring. The protein HER2 is a prognostic biomarker for breast cancer.
Monitoring biomarkers help recognize the status of disease or side effects of treatment. Tumor DNA (ctDNA) is a monitoring biomarker for certain metastatic cancers.
Predictive biomarkers signal the likelihood a person has to develop a reaction after exposure to a disease, treatment, or substance in the environment. Presence of the hormone ER (ER+) in a breast tumor is a predictive biomarker that tamoxifen may be an effective treatment.
Credit: Spiceworks.com
“The Internet of Things presents a tremendous opportunity for developing wearable sensor technologies,” Gao says. “We’ve integrated and miniaturized analytical tools into body-worn platforms, or soft robots as some people call them, to create wireless health monitors.”
Gao’s sweat sensors are soft, flexible nanorobots on the skin that can both stimulate sweat and analyze miniscule amounts of it.
In 2016,when Gao’s lab started presenting wearable devices that had a fully integrated system, their reputation started to grow. His work was featured on the cover of multiple medical engineering journals and even on the cover of Newsweek. His significant work has been described as a critical turning point in the research about sweat sensors, prompting the research on these wearables to grow rapidly.
Gao’s sweat devices collect live data continuously, wirelessly, and outside the lab, at the point of patient care. His sweat sensors are also the first to measure drugs as the biomarker.

This device was designed to measure metabolites in sweat (in a study not funded by ACS). It has a T sensor to measure temperature, as well as the sweat sensors. On the arm, the flexible printed circuit board is under the sensor array.
The layers of these elegantly designed components within a Gao sweat sensor is a practical demonstration of the IoT.
A miniaturized electronic sweat-induction module. “We learned several years ago that having patients exercise was not practical, so we came up with a way to get sweat that didn’t require it,” Gao says. Using a process known as iontophoresis, they apply a very small electric current through the skin to make it more permeable to medication. Gao’s lab uses the drug carbachol to prompt localized sweating by stimulating a sweat gland.
“We only need to do this stimulation in a small area for a few minutes for the sweating process to last hours or even days,” he says. That way researchers can continuously analyze sweat in real time without patients feeling anything. They can even analyze it while people are sleeping.
A microfluidic sweat sampling module. Microfluidics is technology that can manipulate teeny amounts of liquid. Their channels and inlets are less than a quarter of a millimeter wide. That’s not a lot of sweat. This technique allows sweat to be analyzed by the sensor in real time while minimizing evaporation and skin contamination.
The Gao microfluidic design is less expensive than previous versions. The modules are created by engraving special plastic sheets with a carbon dioxide laser, a device so readily available that some hobbyists use it for engraving. Layers of channels are assembled and attached to the sensors.
Electrochemical biosensor. As freshly supplied sweat flows through the microchannels, the sensor can capture changes in the concentration of biomarkers over time. The specific design of the sensor depends on the biomarker(s) being studied. Gao’s advanced miniaturized wearables are multiplexed, meaning they allow for the integration of several sensors to detect different biomarkers from the same sweat sample. They’re also multimodal, as they allow for sensors to detect physical biomarkers (vital signs), including heart and respiratory rates, blood pressure, and temperature. So, although the amount of sweat analyzed and the components are very small, a lot of data is collected. The sensors can recalibrate their readings in real time.
Many molecules that Gao studies—circulating metabolites, nutrients, hormones, proteins, and drugs—cannot be sensed by the existing wearable sensing strategies. Detecting these types of molecules can be realized using bioaffinity receptors such as artificial antibodies (based on molecularly imprinted polymer), which use shape recognition to bind to the biomarker being measured. Because the device allows all the analysis to take place on the skin—not separately in a lab—it’s called in situ detection.
“Our sensors automatically reset right on the skin, so they have a continuous energy source and don’t need batteries like some sensors do,” Gao says.
Printed flexible circuit board. “We can design our own flexible printed circuit board, we also collaborate with some professors who can make the integrated circuit, who can make all our systems even smaller,” Gao says. The board allows for a rapid, real-time sensor readout and was designed and developed to acquire and process data from the sensor and transmit it wirelessly to a mobile phone via Bluetooth communication. The researchers can apply machine learning in the desktop, phone app, or the board.
Skin-worn wearable devices. The devices are attached to the body with skin-friendly, soft and stretchable materials. Gao’s lab has designed and tested sweat sensors that can be worn against the skin (as patches, watches, and headbands). He could also use saliva sensors that are worn on a mouth guard, and tear sensors worn on contact lenses.
Having a compact, functional sensor design allowed the Gao lab to start collaborating with other researchers and clinicians for translational research—the research that takes what was learned in the lab to the patient. See part 2 about sweat sensors to learn about Gao’s collaboration with an oncology team to test sweat sensors with certain blood cancer patients.
Gao’s wearable biochemical sensors offer a real-time, non-invasive tool as an alternative to traditional blood analysis in a lab. “Our devices have the potential to one day allow for at-home personalized health monitoring with significantly reduced costs,” Gao says. “The large sets of data collected by wearable sensors, coupled with modern data analysis—like applying artificial intelligence and machine learning—opens the door for discovering new biomarkers that can keep expanding precision medicine.”
- Helpful resources
- For researchers
American Cancer Society news stories are copyrighted material and are not intended to be used as press releases. For reprint requests, please see our Content Usage Policy.




