Future Defenses Against Colorectal Cancer May Include a Vaccine and Genetic Engineering
Thanks to increased screening rates and improvements in early detection and treatment, the death rate for colorectal cancer has been dropping for decades. Still, colorectal cancer, which includes cancers that start either in the colon or the rectum, is the second leading cause of deaths from cancer when men and women in the US are combined. A little more than 4% of men and women in the US will develop colorectal cancer at some point in their lifetime.
To help lower both the death and diagnosis rates—and to help those who develop colorectal cancer survive and thrive—the American Cancer Society (ACS) funds the research of scientists across the country who use the latest evidence and cutting-edge technology to help prevent and treat CRC. Here are two of their stories.
Working on the First Vaccine to Prevent Colorectal Cancer
There are several FDA- approved vaccines that help protect against viruses that are known to cause certain types of cancer. For example a vaccine against human papillomavirus (HPV) helps protect against 6 types of cancers, and the hepatitis B (HBV) vaccine helps protect against certain types of liver cancer. There isn't a vaccine to protect against colorectal cancer.
Mary L. Disis, MD, is using her 5-year ACS Clinical Research Professor Award to further her efforts toward developing a vaccine that could stop colorectal cancer before it starts.

Nora Disis, MD, professor of medicine at the University of Washington in Seattle, and her daughter at the finish line of the Bra Dash to benefit breast cancer research.
Creating a vaccine to prevent cancer itself has its own set of difficulties, Disis says. When a virus enters the body, it’s fairly easy for the immune system to recognize it as a foreign invader and attack it. But the immune system has a harder time knowing a cancer cell is a foreign invader because it’s often not as easy to recognize the part of it that’s not normal.
In a cancer cell, the proteins look very similar to healthy proteins, so the immune system doesn’t know to attack them.”
“In a cancer cell, the proteins look very similar to healthy proteins, so the immune system doesn’t know to attack them,” she said.
In earlier research, Disis found that breaking a cancer cell’s protein into smaller pieces allowed her to identify certain fragments that the immune system could recognize as foreign. “Those are the fragments we’ve brought into our vaccine in the lab,” she said.
Disis and her team tested their vaccine in mice. “We found if we give the vaccine before giving mice a chemical that caused the development of polyps (and eventually colon cancer), we could prevent 80% of the polyps from becoming colon cancer,” Disis said. In another trial, with mice whose genes had been changed to make them develop polyps and then colon cancer, the vaccine was able to prevent 50% from developing the pre-cancerous polyps, she said.
Right now, she’s preparing an application to the Food and Drug Administration (FDA) to study the vaccine in humans. “In a clinical trial, our plan is to first give the vaccine to people who have a high risk for colorectal cancer,” Disis said. “Then, if over time, we’re able to provide enough evidence about its long-term safety, in the future, the vaccine has the potential to become more widely available. Our dream is to make colorectal cancer largely a disease of the past.”
Using Genetic Engineering to Develop Better Treatments
Lukas Dow, PhD, once thought he’d head back home to Australia after he finished his postdoctoral fellowship. Instead he decided to stay in the US and start his own cancer research lab at Weill Cornell Medicine in New York City, where he’s an assistant professor. Dow is studying why some cancer cells respond well to certain drug therapies while others don’t. He’s specifically looking at a subtype of colorectal cancer that involves a defect where two unrelated genes fuse together. It’s called an RSPO fusion.
Dow says only a small percentage of people with colorectal cancer have this defect. “But because about 1.8 million people around the world are diagnosed with colorectal cancer each year, tens of thousands of people could be affected,” he said.
When the genes fuse together in RSPO fusion, it increases the activity of a family of proteins called WNTs, which help tumors grow. That’s why researchers are developing drugs that kill cancer cells in these tumors by blocking the production of the WNT proteins. Some of these WNT protein-blocking drugs have been tested in phase 1 trials, the first level of human clinical trials, which test the safety of new treatments.
But Dow’s team recently discovered that these drugs don’t permanently stop the tumor’s growth. “My lab found that WNT-targeting drugs cause an immediate and dramatic lessening of disease, but the tumors can grow back when treatment stops,” he said.
“We predicted that a significant number of patients in clinical trials would develop drug resistance and tumor relapse,” Dow said. “We didn’t want to wait until we saw that. So, we’re trying to solve the problems proactively rather than waiting for them to arise.” The research team believes having treatments that are more precise and long lasting may improve many lives.
We use mice to explore how the tumor grows and responds to drugs in a whole organism, and we use cells from biopsies to make miniature intestines in the lab that are called 3D organoids."
Since the specific gene fusion is fairly rare, his group has had to find creative ways to study this cancer. They use mice to explore how the tumor grows and responds to drugs in a whole organism, and they use cells from biopsies to make miniature intestines in the lab that are called 3D organoids. In both approaches, his team uses a genetic engineering tool called CRISPR to precisely edit the DNA to mimic the fusion defect that contributes to the formation of some colon cancers.
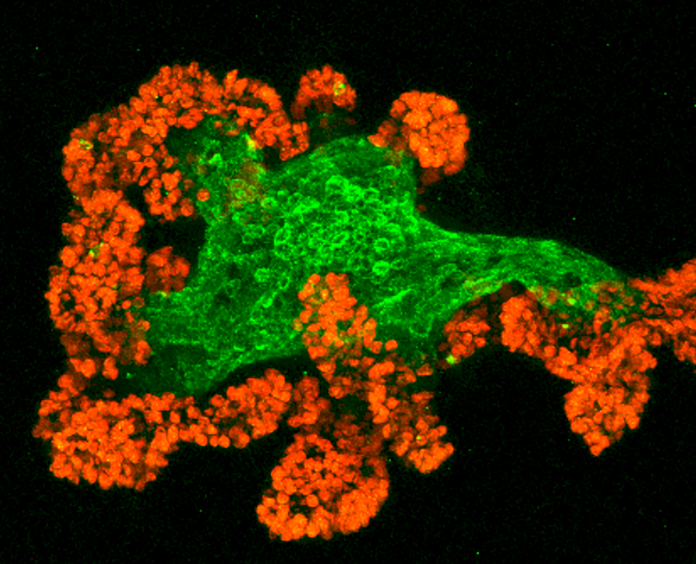
This is a microscopic image of a mouse intestinal organoid that Lukas Dow, PhD, genetically engineered to study a subtype of colorectal cancer in his lab at Weill Cornell Medicine.
“As far as we know, we’re the first people to use genetic engineering tools and technologies to study this subtype of colorectal cancer,” Dow said. “With CRISPR, we can create single or multiple gene mutations, and it takes a lot less time than traditional methods do just to study a single gene.”
“When the edited genes did lead to colon cancer in the mice, we knew we had the first evidence that this specific fusion can drive tumor development,” Dow said.
Dow will also transplant some organoids into mice to see how the cells and structures around the intestines, called the microenvironment, affect the tumor’s response to the WNT-targeting drug. His lab tests how all three RSPO-fusion models—the genetically engineered mice, the organoids, and the two together, mice with a transplanted organoid—react to drug so they can better pinpoint who might benefit most from WNT-targeted drugs. They’re also looking for additional treatments that would help prevent or overcome drug resistance.
“There are multiple, upcoming clinical trials for RSPO-related tumors, and we believe our work could have an immediate and significant impact.”



