Your gift is 100% tax deductible.
Breast Reconstruction Using Your Own Tissue (Flap Procedures)
A tissue flap procedure (also known as autologous tissue reconstruction or tissue-based reconstruction) is one way to rebuild the shape of your breast after surgery to remove the cancer. As with any surgery, you should learn as much as possible about the benefits and risks, and discuss them with your doctor, before having the surgery.
Advantages of tissue flaps
These procedures use tissue from other parts of your body, such as your tummy, back, thighs, or buttocks to rebuild the breast shape. Tissue flaps look and feel more natural and act more like natural breast tissue than breast implants. Unlike implants, tissue flaps will change like any other tissue in your body. For instance, they may get bigger or smaller as you gain or lose weight. And while breast implants sometimes need to be replaced (if the implant ruptures, for example), this is not a concern with tissue flaps. Tissue flaps are often used by themselves to reconstruct the breast, but some tissue flap procedures can be used with a breast implant if more volume is needed.
Disadvantages of tissue flaps
Tissue flap procedures can also have some downsides that need to be considered:
- In general, flaps require more surgery and a longer recovery time than breast implant procedures
- Flap operations leave 2 surgical sites and scars – one where the tissue was taken from (the donor site) and one on the reconstructed breast. The scars fade over time, but never go away completely
- Some women can have donor site problems such as abdominal bulging, muscle damage or weakness, and contour distortions such as dimpling of the skin
Types of tissue flap procedures
There are many different types of flap procedures. They are often named by the muscle or artery that is being used and they mainly fall in two groups:
Pedicle flaps: A pedicle flap moves tissue from its site to the breast or chest wall while it is still attached to its original blood supply. The most common pedicle flap used for breast reconstruction is the latissimus dorsi (LD) flap, where tissue from the back (skin, fat, and muscle) is used to make a new breast. Tissue from the abdominal wall (tummy) can also be used as a pedicle flap (transverse rectus abdominis muscle or TRAM flap). But this has been largely replaced by its free flap version, where the muscle can be totally or partially saved.
Free flaps: A free flap moves tissue, fat, skin, and some or none of the muscle from one area of the body to make a new breast. This tissue is completely removed from the body and moved up to the chest. The blood vessels (arteries and veins) must then be reconnected to the chest wall vessels for the tissue to survive. This requires the use of a microscope (microsurgery) to connect the tiny vessels, and the surgery takes longer than a pedicle flap. Most of the time, free flaps don't need to take the muscle from the donor site, so there is less risk of losing muscle strength, and the donor site often looks better than if the muscle had been removed. The main risk is that sometimes the blood vessels get clogged and the flap doesn’t work because of poor or no blood supply. The abdominal wall (tummy) is the most popular and common donor site for free flap breast reconstruction. Other possible donor site areas for breast free flap reconstruction are the thighs, buttocks, and lower back.
Restoring feeling to the reconstructed breast
During a mastectomy, nerves are cut causing a loss of sensation (feeling) on that side. The skin on the chest wall can feel numb (no feeling) or be more sensitive. The feeling might return after a few months or years or not at all. Finding ways to restore the feeling in the reconstructed breast has become a goal of tissue (flap) breast reconstruction. It is often possible to keep a sensory nerve (a nerve that controls feeling) within the flap. On the chest wall, a nerve in between the ribs is isolated and then reconnected with the nerve of the flap. This connection helps stimulate the tissue flap to regain feeling. There are studies that show improvement of sensation using this technique.
Abdominal (tummy) flaps
An abdominal wall flap procedure uses tissues from the tummy. Most times the tummy provides enough tissue for breast reconstruction, so no breast implants are needed. The tummy flap names are based on how the tissue is transferred and if the abdominal wall muscle is used or not. The donor site of the abdominal wall flap may look like a “tummy tuck,” but it can also reduce the strength in your belly muscles and cause bulging depending on what technique was used. Tummy flaps may not be possible in women who are very thin or who have had a tummy tuck before.
There are different types of abdominal wall (tummy) flaps:
- A pedicle transverse rectus abdominal muscle (TRAM) flap leaves the flap attached to its original blood supply and tunnels it under the skin to the chest. It usually requires removing most if not all of the rectus abdominis (6-pack) muscle on that side, which means an increased risk of bulging on one side of the abdomen. This can also mean your abdominal (belly) muscles may not be as strong as before the surgery.
- A free TRAM flap moves tissue and most, if not all, of the muscle) from the same part of the lower abdomen as a pedicle TRAM flap, but the flap is completely removed and moved up to the chest. The blood vessels (arteries and veins) must then be reattached. A microscope is required to connect the tiny vessels (microsurgery), and the surgery takes longer than a pedicle TRAM flap. The main advantage of a free TRAM flap is that the blood supply to the flap is usually better than with a pedicle TRAM flap. The main risk of free flaps is that sometimes the blood vessels get clogged and the flap doesn’t work, but this is rare. There is also a higher risk of abdominal wall weakness and bulging.
A free muscle-sparing TRAM (MS-TRAM) flap is like a free TRAM flap except only part of the muscle from the same part of the lower abdomen, is completely removed and moved up to the chest. The blood vessels (arteries and veins) must then be reattached with microsurgery. Here the plastic surgeon saves most of the abdominal wall muscles; only a small piece of muscle is taken with the flap. There is less risk of abdominal wall bulging and losing abdominal muscle strength, and the donor site (abdomen) often looks better.
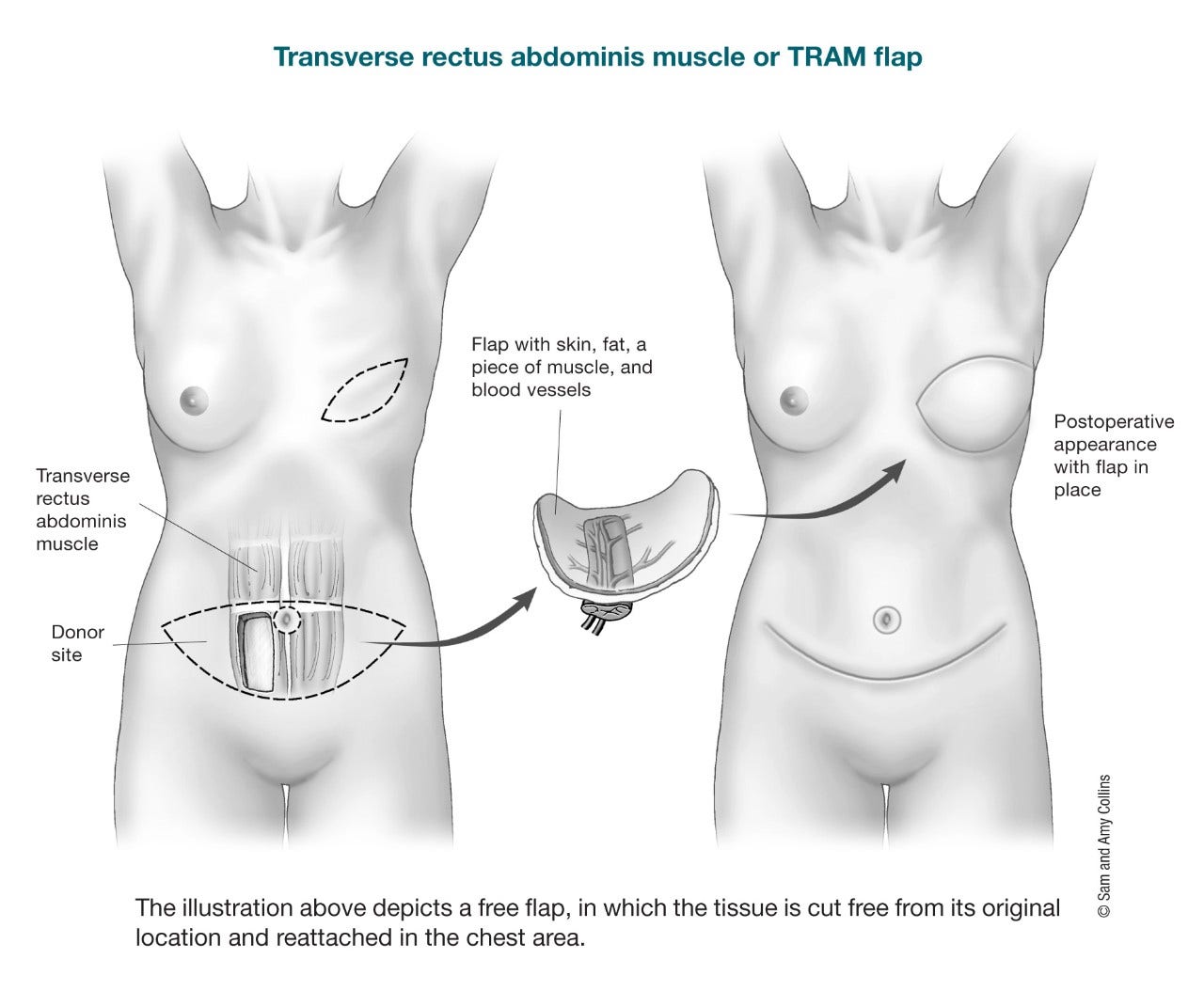
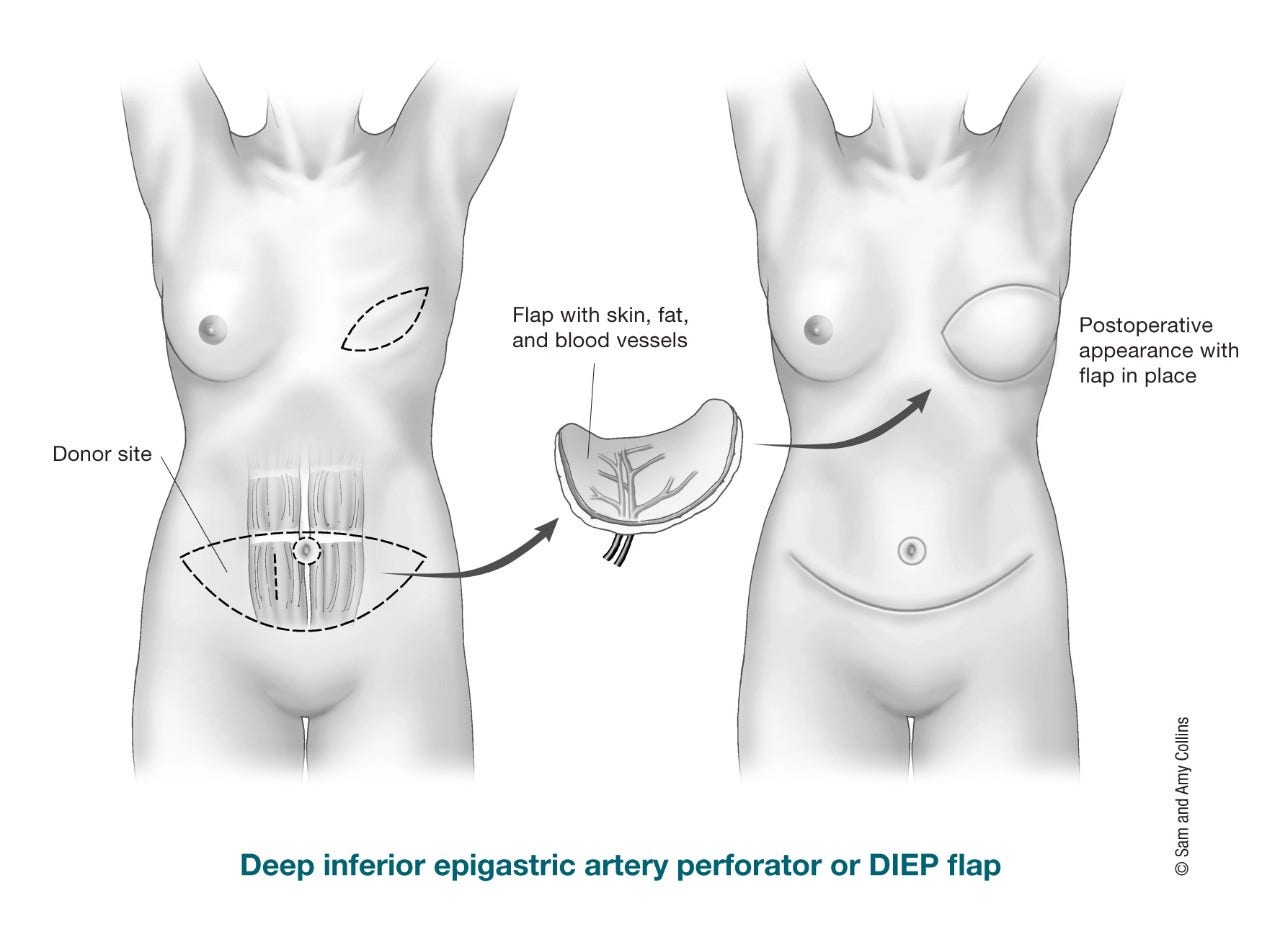
The DIEP (deep inferior epigastric perforator) free flap uses fat and skin from the same area as the TRAM flap to form the breast shape. The difference compared to a free TRAM flap is that no muscle is taken from the abdominal wall. The tissue is completely cut free from the tummy and then moved to the chest. As in the free TRAM flap surgery, a microscope is needed to connect the tiny blood vessels. There’s much less risk of a bulge because no muscle is taken. The free MS-TRAM flap and DIEP free flap are very similar to each other; in one (MS-TRAM flap) you just take a small piece of muscle, and in the other one (DIEP flap) no muscle.
Another possible abdominal wall (tummy) flap is the SIEA (superficial inferior epigastric artery) free flap. Basically, it uses the same tissues as the TRAM and DIEP flaps, but different blood vessels. The blood vessels used for the SIEA flap are more superficial (shallow) and not every person has them. Very few people are candidates for a SIEA flap.
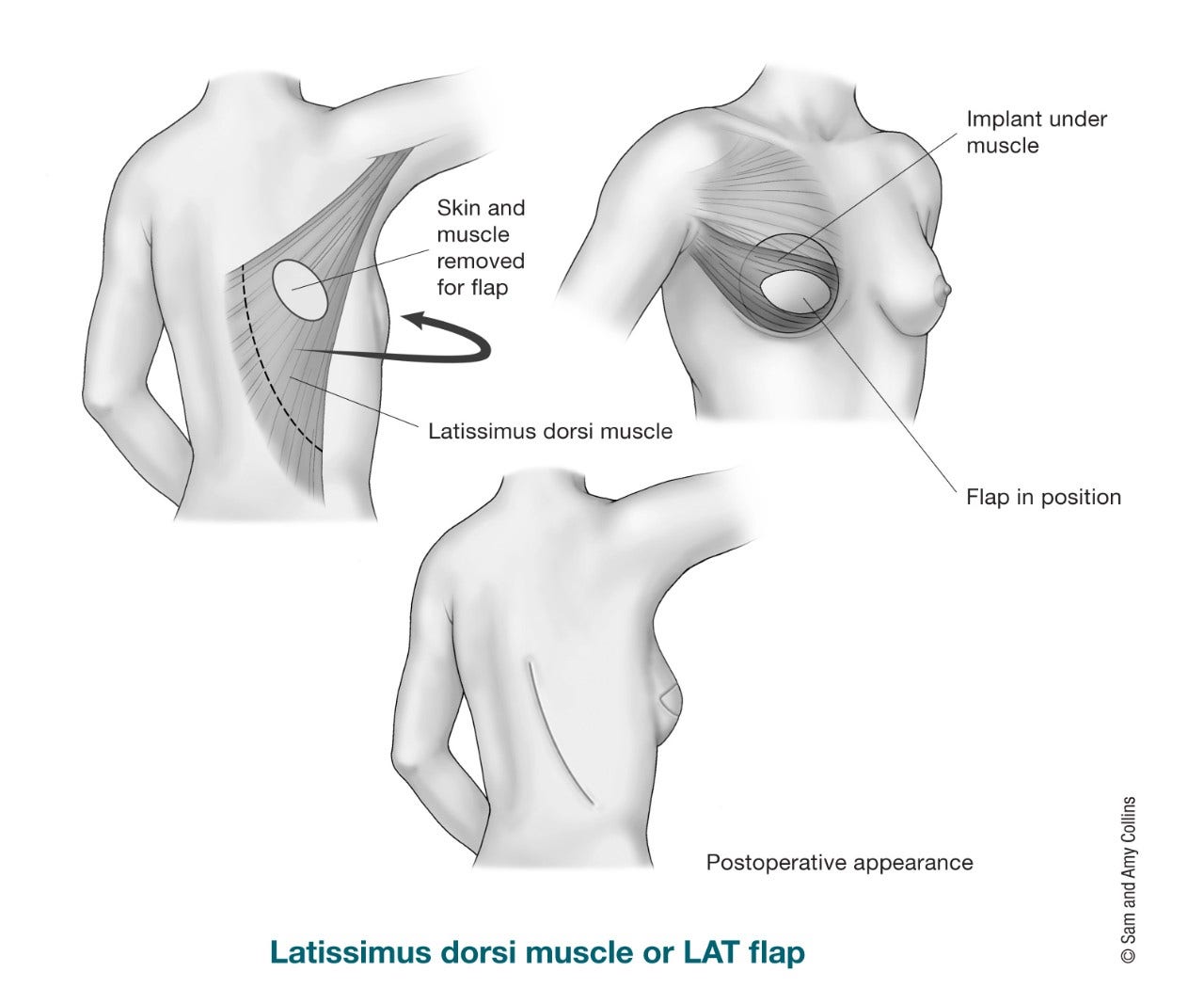
Back flaps
The latissimus dorsi flap is a pedicle flap used for breast reconstruction. Since there is usually not as much tissue there as from an abdominal wall (tummy) donor site, this type of flap is often used with a breast implant to add volume (size) to breast reconstruction. For this procedure, the surgeon tunnels muscle, fat, skin, and blood vessels from your upper back, under the skin to the front of the chest. This type of reconstruction can sometimes be used without an implant depending on the amount of tissue and the desired breast size. Even though one muscle from the back is taken with the flap, rarely do women have weakness in their back, shoulder, or arm after this surgery.
There are also pedicle back flaps that do not take any muscle. The thoracodorsal artery perforator (TDAP) flap takes skin and fat from the upper back, but does not take any muscle. It is usually used for reconstruction after lumpectomy or partial mastectomy when needed.
A newer type of procedure, called a lumbar artery perforator (LAP) free flap, might be an option if there is not enough abdominal wall (tummy) tissue to use as a donor site . The skin, fat, and blood vessels are removed from the lower back area (also sometimes called “love handles”) and moved to the chest and the blood vessels are reconnected. No muscle is removed. The LAP free flap can only be done on one side at a time (one breast at a time), has an extra step to reconnect the blood vessels, and it is offered only at a few hospitals in the US.
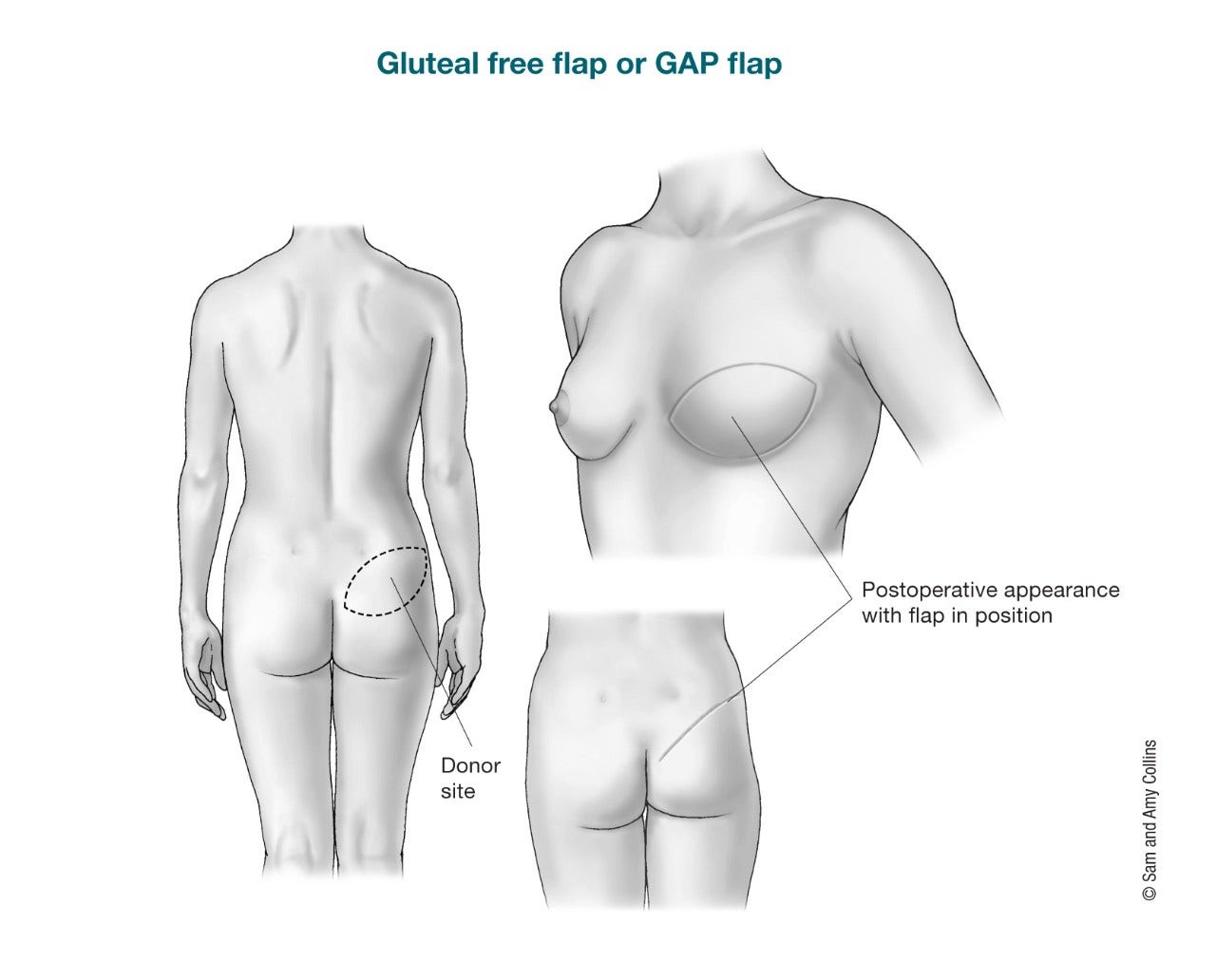
Buttock (bottom) flaps
The gluteal free flap or gluteal artery perforator (GAP) flap uses tissue from the buttocks (bottom) to create the breast shape. The gluteal free flap might be an option for women who cannot or do not wish to use the tummy site because they are thin, have previous incisions, have had a previous tummy tuck, or for other reasons, but it’s not offered at all surgical centers. The skin, fat, and blood vessels are cut out of the buttocks and then moved to the chest. No muscle is removed. The blood vessels will also be reattached. You might see this type of flap called a superior gluteal artery perforator (SGAP) flap if the artery in the upper buttocks is used. The IGAP flap (inferior gluteal artery perforator flap) is a similar surgery except the artery in the bottom part of the buttocks is used. The major drawback of this flap is the possible change in the buttock contour, such as skin dimpling. For this reason, it has not become very popular.
Thigh flaps
If tissue from the abdominal wall (tummy) cannot be used, the tissues in the thighs are often looked at for breast reconstruction.
Depending on a women's body build and preferences, there are good free flap options from tissues of the inner and outer thighs. All the options require the use of microsurgery and reconnection of the blood vessels in the chest. Most of the time, the thighs only provide enough tissue to make a small or medium-sized breast. In some cases, two flaps, each one from a different thigh, can be used to reconstruct one breast.
Inner thigh: The main options for free flaps from the inner thighs are.
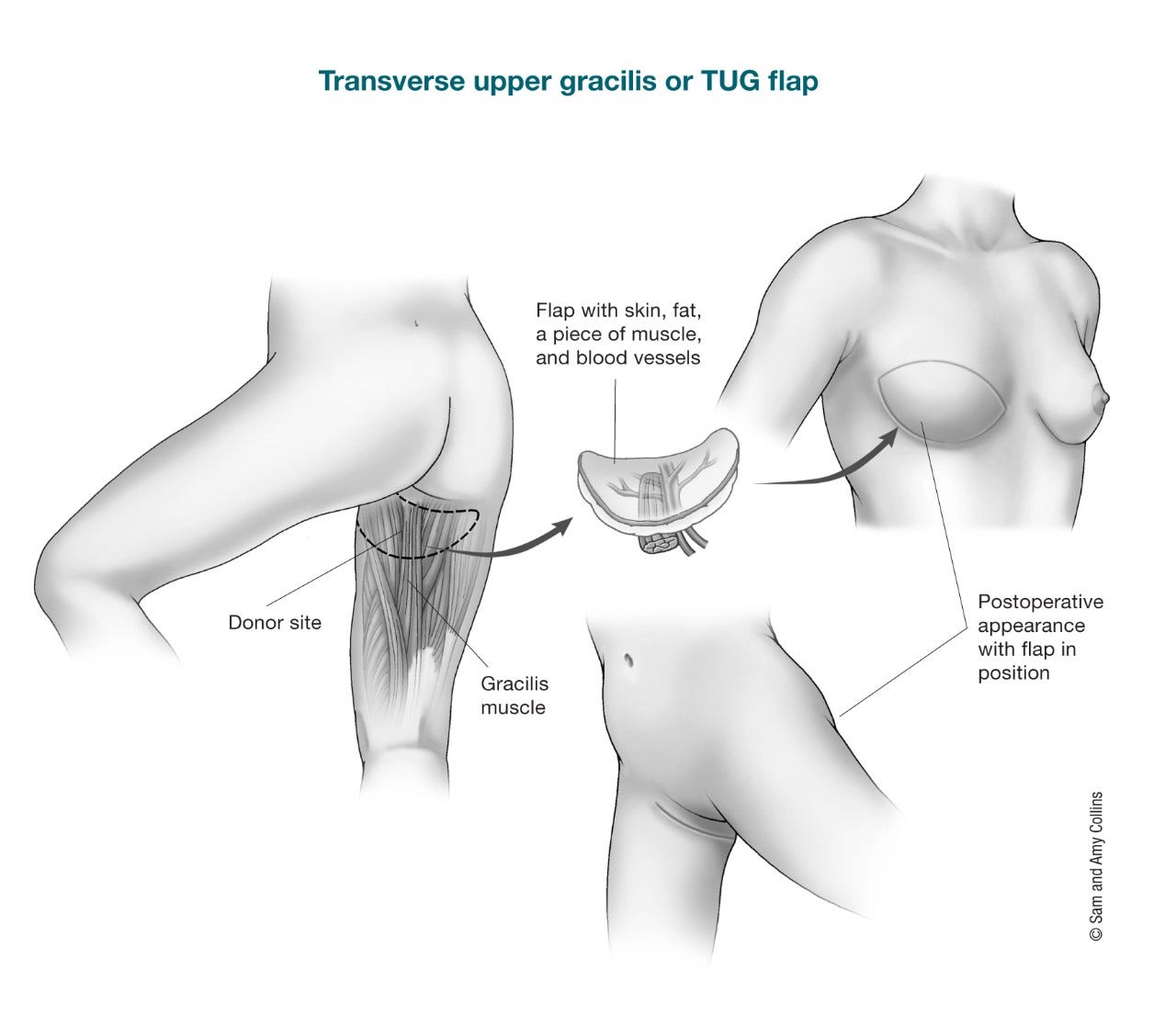
- Upper Gracilis flap: Here skin, fat, and part of the gracilis muscle are taken to make a new breast. Depending on the shape and which part of the muscle is used, it can be called a transverse upper gracilis (TUG) flap, vertical upper gracilis (VUP) flap, or diagonal upper gracilis (DUG) flap.
- Profunda artery perforator (PAP) flap: This flap only takes skin and fat. No muscle is removed. It can also be called horizontal, vertical, or diagonal. This flap has gained popularity lately. It spares a muscle from the donor site and the vessels are easier to work with.
Outer thigh: Another option for women who might have more fatty tissue on the outer part of their thighs and cannot have or choose not to have a DIEP flap is the lateral thigh perforator (LTP) flap. Also called the “saddlebag” flap. The skin, fat, and other tissue is removed from the area in the upper outer thigh and upper buttock and moved to the chest. No muscle is taken.
Fat grafting
Fat grafting is usually used for breast revisions or “touch up” surgeries. Your own fat is used to help fix any shape abnormalities that may be seen after the initial breast reconstruction surgery is done. The fat is not removed with skin, muscle, or other tissues.
The fat is obtained by liposuction, cleaned and then prepared so it can be injected easily into the areas it is needed. This is an outpatient procedure and you can go home the same day. Often, more then one session of fat grafting is needed to correct some contour deformities. This procedure has been found to be safe as far as cancer recurrence in patients who have had mastectomies.
- Written by
- References

The American Cancer Society medical and editorial content team
Our team is made up of doctors and oncology certified nurses with deep knowledge of cancer care as well as editors and translators with extensive experience in medical writing.
American Society of Plastic Surgeons. Breast Reconstruction. Accessed at https://www.plasticsurgery.org/reconstructive-procedures/breast-reconstruction on July 29, 2021.
Ananthakrishnan P, Lucas A. Options and considerations in the timing of breast reconstruction after mastectomy. Cleve Clin J Med. 2008;75 Suppl 1:S30-33.
Andrades P, Fix RJ, Danilla S, Howell RE 3rd, et al. Ischemic complications in pedicle, free, and muscle sparing transverse rectus abdominis myocutaneous flaps for breast reconstruction. Ann Plast Surg. 2008;60:562-567.
Beugels J, Bijkerk E, Lataster A, Heuts EM, van der Hulst RRWJ, Tuinder SMH. Nerve Coaptation Improves the Sensory Recovery of the Breast in DIEP Flap Breast Reconstruction. Plast Reconstr Surg. 2021;148(2):273-284. doi:10.1097/PRS.0000000000008160.
Beugels J, van Kuijk SMJ, Lataster A, van der Hulst RRWJ, Tuinder SMH. Sensory Recovery of the Breast following Innervated and Noninnervated Lateral Thigh Perforator Flap Breast Reconstruction. Plast Reconstr Surg. 2021;147(2):281-292. doi:10.1097/PRS.0000000000007547.
Bijkerk E, van Kuijk SMJ, Lataster A, van der Hulst RRWJ, Tuinder SMH. Breast sensibility in bilateral autologous breast reconstruction with unilateral sensory nerve coaptation. Breast Cancer Res Treat. 2020;181(3):599-610. doi:10.1007/s10549-020-05645-y.
Boehmler JH and Butler CE. Chapter 5: Latissimus Dorsi Flap Breast Reconstruction. In: Hall-Findlay EJ, Evans GRD, eds. Aesthetic and Reconstructive Surgery of the Breast. W.B. Saunders; 2010.
De La Cruz L, Blankenship SA, Chatterjee A, et al. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: A systematic literature review. Annals of Surgical Oncology. 2016; 23(10):3247-3258.
Djohan R, Gage E, Bernard S. Breast reconstruction options following mastectomy. Cleve Clin J Med. 2008;75 Suppl 1:S17-23.
Farhangkhoee H, Matros E, Disa J. Trends and concepts in post-mastectomy breast reconstruction. J Surg Oncol. 2016;113(8):891–894.
Goodenough CJ, Rose J. Breast Transverse Rectus Abdominus Muscle Procedure. [Updated 2021 Jun 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539759/.
Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32(9):919–926.
Jagsi R, King TA, Lehman C, Morrow M, Harris JR, Burstein HJ. Chapter 79: Malignant Tumors of the Breast. In: DeVita VT, Lawrence TS, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 11th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2019.
Losken A, Pinell-White X, Hodges M, Egro FM. Evaluating outcomes after correction of the breast conservation therapy deformity. Ann Plast Surg. 2015 Jun;74 Suppl 4:S209-13.
Mehrara BJ, Ho AY. Breast Reconstruction. In: Harris JR, Lippman ME, Morrow M, Osborne CK, eds. Diseases of the Breast. 5th ed. Philadelphia: Wolters Kluwer Health; 2014.
Nahabedian MY. Factors to consider in breast reconstruction. Womens Health (2015) 11(3), 325–342.
Nahabedian M. Options for autologous flap-based breast reconstruction. In Collins KA, ed. UpToDate. Waltham, Mass.: UpToDate, 2021. https://www.uptodate.com. Accessed August 2, 2021.
National Cancer Institute. Breast Reconstruction After Mastectomy. 2017. Accessed at https://www.cancer.gov/types/breast/reconstruction-fact-sheet on August 2, 2021.
National Comprehensive Cancer Network (NCCN). Practice Guidelines in Oncology: Breast Cancer. Version 5.2021. Accessed at https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf on July 29, 2021.
Patel K, Bloom J, Nardello S, Cohen S, Reiland J, Chatterjee A. An Oncoplastic Surgery Primer: Common Indications, Techniques, and Complications in Level 1 and 2 Volume Displacement Oncoplastic Surgery. Ann Surg Oncol. 2019 Jul 24. doi: 10.1245/s10434-019-07592-5. [Epub ahead of print]
Rose J, Puckett Y. Breast Reconstruction Free Flaps. [Updated 2021 Jun 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541048/.
Spiegel AJ, Menn ZK, Eldor L, Kaufman Y, Dellon AL. Breast Reinnervation: DIEP Neurotization Using the Third Anterior Intercostal Nerve. Plast Reconstr Surg Glob Open. 2013;1(8):e72. Published 2013 Dec 6. doi:10.1097/GOX.0000000000000008.
Thomsen JB, Rindom MB, Rancati A, Angrigiani C. Thoracodorsal artery flaps for breast reconstruction-the variants and its approach. Arch Plast Surg. 2021;48(1):15-25. doi:10.5999/aps.2020.01410.
Upadhyaya SN, Bernard SL, Grobmyer SR, Yanda C, Tu C, Valente SA. Outcomes of Autologous Fat Grafting in Mastectomy Patients Following Breast Reconstruction. Ann Surg Oncol. 2018 Oct;25(10):3052-3056.
Vincent A, Hohman MH. Latissimus Dorsi Myocutaneous Flap. [Updated 2021 Feb 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564377/.
Last Revised: October 20, 2021
American Cancer Society medical information is copyrighted material. For reprint requests, please see our Content Usage Policy.
American Cancer Society Emails
Sign up to stay up-to-date with news, valuable information, and ways to get involved with the American Cancer Society.



