Your gift is 100% tax deductible.
Preserving Your Fertility When You Have Cancer (Women)
If you are a woman (or if you have female reproductive organs), cancer and cancer treatment could affect your ability to have a child. Before you start treatment, talk with your doctor and cancer care team about how your treatment might affect your fertility.
There may be ways to improve your chances of having children in the future.
Having a child after cancer treatment
Certain types of cancer treatment could cause you to become infertile (unable to become pregnant or carry a baby to full term). For some women*, fertility returns after treatment is finished. But some women lose their fertility and never regain it.
You might be able to take steps to help preserve your fertility. Fertility preservation saves or protects your embryos, eggs, or ovarian tissue so you can use them to have children in the future.
If you’re interested in fertility preservation, you should talk to your health care team before your cancer treatment begins.
*Learn more about the gender terms used here and how to start the conversation with your cancer care team about your gender identity and sexual orientation in Gender Identity, Sexual Orientation, and Fertility.
Talking with your health care team about fertility
You might need to start the conversation about fertility preservation. Don't assume your doctor or cancer care team will ask. They don’t always remember to bring this up, so be prepared to bring it up yourself.
This is especially true if you are part of the LGBTQ+ community. Studies show that many doctors and nurses don't know the right questions to ask.
You can learn more about how you can start the conversation with your health care team in How Cancer and Cancer Treatment Can Affect Fertility in Women.
Possible pregnancy without fertility assistance
If you were fertile before treatment, it’s possible your body might recover afterward. Your normal hormonal cycles may begin again, and your body may be able to produce mature eggs. This could allow you to get pregnant through sexual intercourse.
But if you go through chemo or have radiation to your pelvis, you could be at risk for sudden, early menopause. This could happen even if your start having menstrual cycles again after treatment. Menopause could start 5 to 20 years earlier than expected.
It’s important to talk with your cancer care team before making a pregnancy plan. They may also recommend you wait a certain amount of time before trying to get pregnant. This wait time could be as much as 2 years.
- A wait time of 6 months might reduce the risk of birth defects from eggs damaged by chemotherapy or other treatments.
- A wait time of 2 years is because the risk of the cancer coming back (recurring) is usually highest in the first 2 years after treatment.
The length of time you’ll need to wait depends on the type of cancer you have and the treatment you get. Talk to your cancer care team about how long you should wait and why.
Understanding Your Options
As you explore your options for fertility preservation, be sure you understand the risks and chances of success. Keep in mind that no method works 100% of the time.
Experts suggest that all members of the cancer care team should be involved in talking with women about their fertility. This might include medical oncologists, radiation oncologists, gynecologic oncologists, hematologists, pediatric oncologists, surgeons, nurses, social workers, navigators, and others.
Your cancer care team should talk to you about any possible fertility problems you might have because of treatment. This conversation should happen as early as possible, before treatment starts. These conversations should consider your preferences, religious or personal beliefs, and the cost of available options.
You might want to get a second opinion. You can also ask for a referral to a fertility or reproductive specialist.
Cryopreservation (freezing embryos or eggs)
Cryopreservation can help preserve fertility for some women with cancer. During cryopreservation, embryos, eggs, or parts of an ovary are taken out and frozen.
It's important to find a fertility specialist and center that has experience with these procedures. Talk to your cancer care team about seeing a fertility specialist. Also, make sure to ask your team if they have any concerns about delaying your cancer treatment until after you complete cryopreservation.
Embryo freezing
Embryo freezing (embryo cryopreservation) is one option to help preserve fertility for women getting treated for cancer. During embryo freezing, mature eggs are removed from your ovaries and fertilized in one of two ways:
- In vitro fertilization (IVF): The egg is put in a sterile lab dish with several thousand sperm. The goal is for one of the sperm to fertilize the egg.
- In vitro intracytoplasmic sperm injection (IVF-ICSI): A single sperm is injected directly into an egg to fertilize it.
For both procedures, the lab dish is watched to see if the egg is fertilized. If it is, the embryo can be frozen. Then, after cancer treatment ends and you are ready to try to get pregnant, the embryo is thawed and placed into your uterus.
How egg retrieval works
To get mature eggs, your ovaries will need to be stimulated with medicines. It can take 2-3 weeks for this to work.
Once the eggs seem mature, they are retrieved through your vagina. A transvaginal ultrasound is placed into your vagina so the doctor can see your ovaries. A needle is then passed through the vagina into the ovaries to pull out the liquid that has the eggs. You will get medicine to make you sleepy for this.
How your age affects embryo freezing
Your age can affect your chance of getting pregnant this way. If you are getting close to menopause, this can also affect your chance of success. Women who are younger at the time of egg retrieval are more likely to get pregnant. Also, the health of the embryos makes a difference. Some embryos may not survive the thawing process. Some may not implant into the uterus correctly.
Egg (oocyte) freezing
Egg freezing (oocyte cryopreservation) is another option to help preserve fertility. This may be a good choice if you don’t have a partner and don’t want to use donor sperm to make a fertilized embryo, or if you aren’t comfortable with freezing a fertilized embryo.
For egg freezing, mature eggs are removed from your ovaries and frozen without being fertilized. This process is sometimes called egg banking. When you are ready to try to get pregnant, your eggs are thawed and fertilized by a partner's or donor's sperm. The fertilized eggs are then implanted in your uterus.
The process for stimulating the ovaries and retrieving the eggs is the same for embryo and egg freezing.
Ovarian tissue freezing
This involves taking out all or part of an ovary and freezing it. The ovary is removed with surgery, often through a laparoscope. This is a minor surgery where a thin, flexible tube is passed through a small cut near your navel. With this tube, the surgeon can reach and look into your pelvis.
Once it is removed, the ovarian tissue is usually cut into small strips, frozen, and stored.
After cancer treatment, the ovarian tissue can be thawed and placed back in your body, either on a remaining ovary or near where the ovary was taken from before cancer treatment. Once the ovarian tissue starts to function again, you might be able to get pregnant naturally or you may need IVF.
Ovarian tissue freezing can be done either before or after puberty. This provides an option for children who haven’t yet gone through puberty.
Cryopreservation costs
Cryopreservation can cost $10,000 or more, not including storage fees.
The exact costs vary depending on the procedure you have and where you go to get it done. You’ll need to pay for the procedure to collect and freeze the embryos, eggs, or ovarian tissue. You will also have to pay for storage. This is usually a yearly fee.
When you meet with the fertility specialist, ask what your costs would be for the procedure and storage. Check with your insurance company about whether they cover any of these costs. Also be sure to ask whether they have a preferred provider or location.
If you freeze your eggs, embryos, or ovarian tissue, it’s important to stay in contact with the storage facility to be sure any yearly storage fees are paid and your contact information is up to date.
Ovarian transposition
Ovarian transposition (oophoropexy) is a surgery to move the ovaries away from the radiation field. It can be used either before or after puberty.
This is often an outpatient surgery unless it is being done as part of a larger operation. During the surgery, your ovaries are moved above and to the side of your central pelvic area. It’s usually best to do the procedure just before you start radiation therapy, because the ovaries tend to fall back into their normal position over time.
If your ovaries don’t fall back to their normal position after radiation treatment, you may need another surgery to move them back before you can get pregnant.
Ovarian transposition is successful about half of the time. Because of radiation scatter, the ovaries are not always protected.
Many insurance plans don’t cover the cost of this procedure, but be sure to check with your plan. If the procedure can be done during another surgery, that might lower your costs.
Fertility-sparing surgery
There are surgeries that can be used to treat certain cancers while still protecting a woman’s ability to have children.
For early-stage cervical cancer, the surgeon can sometimes remove the cervix (trachelectomy) without removing the entire uterus or ovaries.
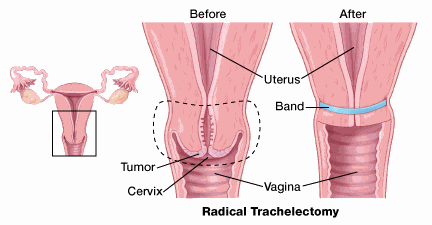
For early-stage ovarian cancer that only affects one ovary, the surgeon might be able to remove the affected ovary and leave the other ovary intact.
Hormone Treatment
Hormones might be used to help preserve your fertility in certain situations.
Hormone therapy for early-stage endometrial cancer
Young women who have early-stage endometrial (uterus) cancer and would like to try to have a child may be able to postpone surgery by having hormone therapy. The hormones used are progestins.
This is a short-term measure. You must be watched closely and checked every 3-6 months to make sure your cancer hasn’t spread. If there is still no cancer after 6 months, you can try to become pregnant. You will continue to be checked for cancer every 6 months.
Because endometrial cancer often comes back, doctors recommend a total hysterectomy and removal of the ovaries and fallopian tubes after childbearing is complete.
Ovarian suppression
For pre-menopausal women, a medicine called GnRH agonist therapy (gonadotropin-releasing hormone agonist therapy) is sometimes used to shut down the ovaries. This may protect their eggs from cancer treatment.
Some studies have suggested that this approach may not be as beneficial as it was once thought. However, there are still situations where ovarian suppression with GnRH is appropriate.
Resources
- Livestrong Fertility: Provides financial assistance to people with cancer who want to preserve their embryos, eggs or sperm. Also offers a fertility clinic search tool.
Learn more
- Written by
- References

Developed by the American Cancer Society medical and editorial content team with medical review and contribution by the American Society of Clinical Oncology (ASCO).
Campbell SB, Woodard TL. An update on fertility preservation strategies for women with cancer. Gynecol Oncol. 2020;156(1):3-5.
Gupta D, Singh S, Shukla S, Shrivastava S. Oncofertility: Treatment options from bench to bedside. Cancer Pathog Ther. 2023;1(4):284-289.
Henry L, Labied S, Jouan C, Nisolle M. Preservation of female fertility: The current therapeutic strategy. Int J Gynaecol Obstet. 2022;156(1):3-9.
Lambertini M, Boni L, Michelotti A, et al. Long-Term Outcomes With Pharmacological Ovarian Suppression During Chemotherapy in Premenopausal Early Breast Cancer Patients. J Natl Cancer Inst. 2022;114(3):400-408.
National Cancer Institute (NCI). Fertility Issues in Girls and Women with Cancer. Accessed at https://www.cancer.gov/about-cancer/treatment/side-effects/fertility-women on August 5, 2024.
Oktay K & Sonmezer M. Fertility preservation: Cryopreservation options. In, UpToDate, Post TW (Ed), UpToDate. Accessed at uptodate.com on August 1, 2024.
Oktay et al. Fertility preservation in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology. 2018;36(19):1994-2003.
Sciorio R. Cryopreservation of human embryos and oocytes for fertility preservation in cancer and non-cancer patients: A mini review. Gynecol Endocrinol. 2020; Jan:1-8.
Society for Assisted Reproductive Technologies. A Patient’s Guide to Assisted Reproductive Technology. Accessed at https://www.sart.org/patients/a-patients-guide-to-assisted-reproductive-technology/ on July 17, 2024.
Sonmezer M & Oktay K. Fertility and reproductive hormone preservation: Overview of care prior to gonadotoxic therapy or surgery. In, UpToDate, Post TW (Ed), UpToDate. Accessed at uptodate.com on August 1, 2024.
U.S. Department of Health and Human Services, National Institutes of Health (NIH). Fertility and infertility. Accessed at https://www.nichd.nih.gov/health/topics/infertility on July 17, 2024.
Last Revised: January 17, 2025
American Cancer Society medical information is copyrighted material. For reprint requests, please see our Content Usage Policy.
American Cancer Society Emails
Sign up to stay up-to-date with news, valuable information, and ways to get involved with the American Cancer Society.



